
ΔLatticeU denotes the molar lattice energy.The molar lattice energy of an ionic crystal in terms of molar lattice enthalpy, pressure, and volume change can be expressed as follows: Read More: Chemical Bonding and Molecular Structure Important Questions The smaller the size of the constituent ions, the greater will be the lattice energy.Small atoms have smaller interatomic distances in the ionic lattice and stronger binding forces.The more the distance between the ions in a lattice, the weaker the electrostatic forces, and the lower will be the lattice energy.Lattice Energy is inversely proportional to the distance between the ions.Hence, it is considered that the lattice energy of CaCl2 is greater than that of KCl.Thus, the electrostatic forces of attraction are stronger in calcium chloride than in potassium chloride.
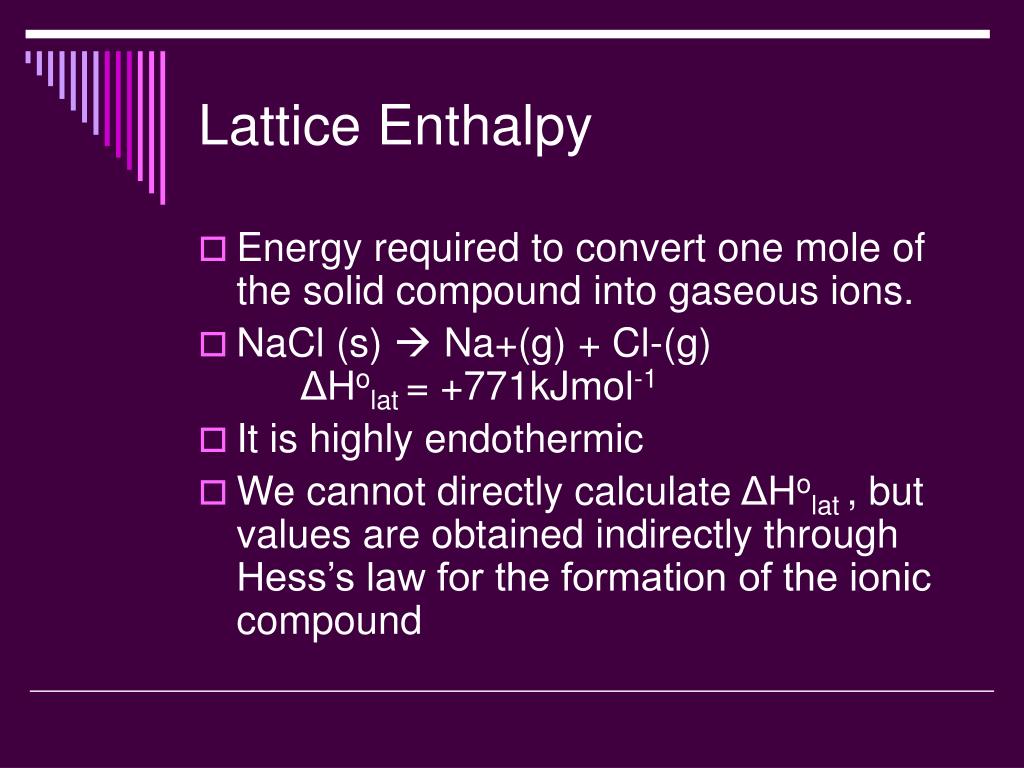
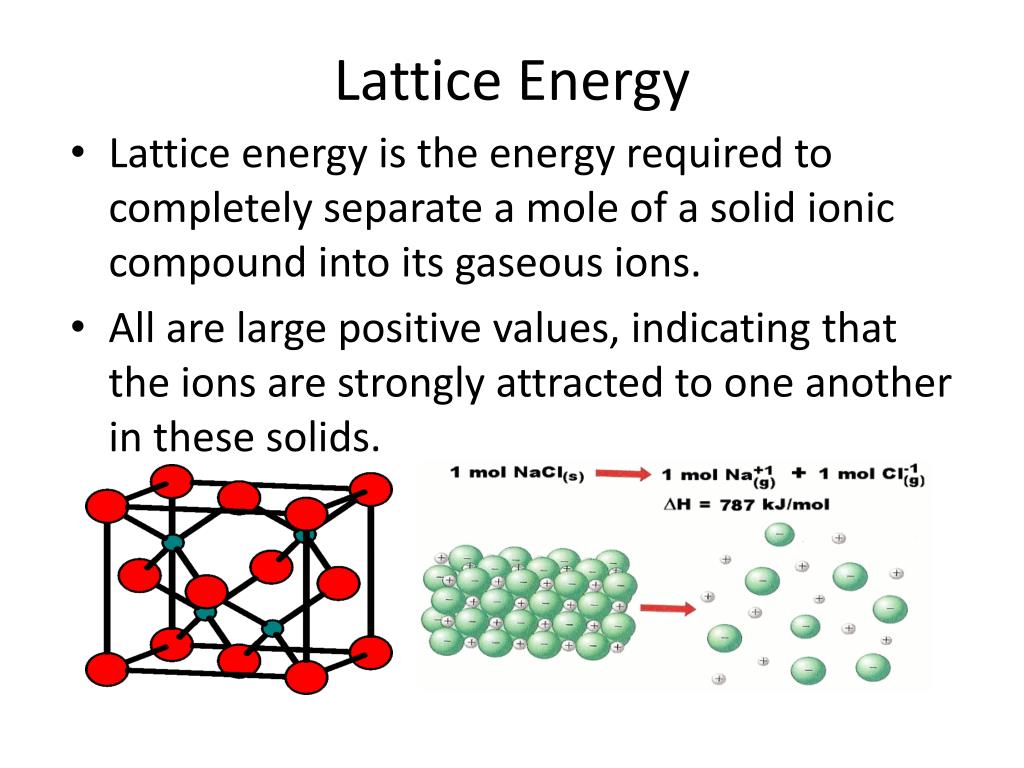
U is the Lattice Energy of an Ionic Compound.Lattice Energy of ionic compounds is calculated using the simplified form of Coulomb’s Law.
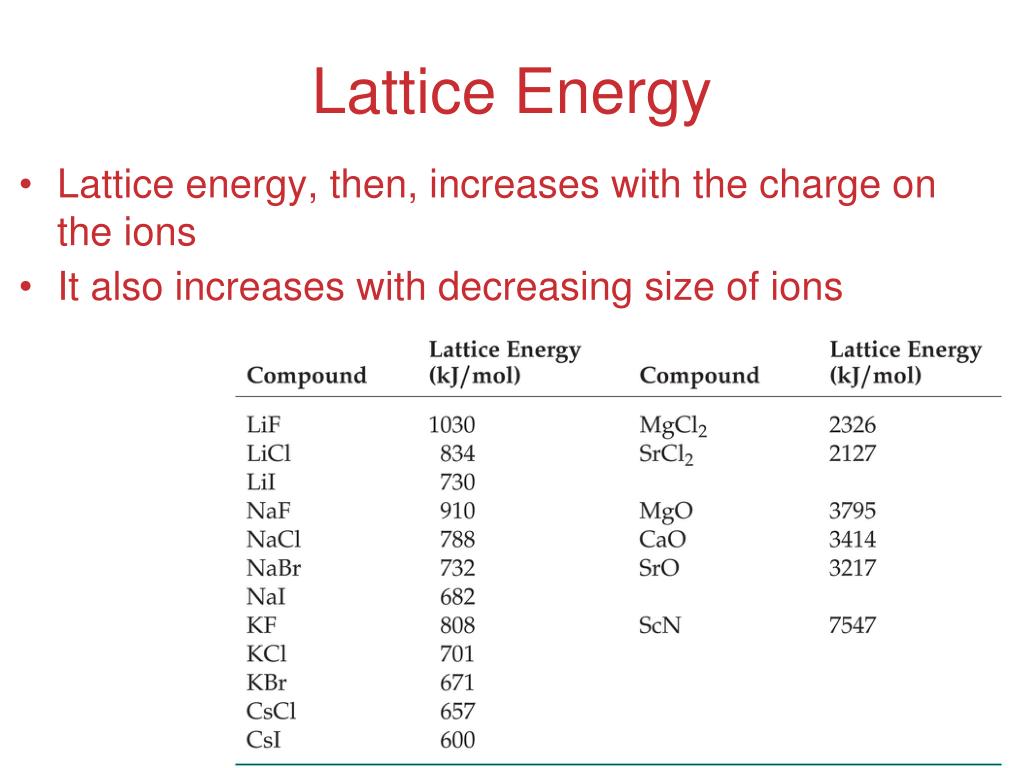
The exact value of the lattice energy of an ionic compound cannot be determined easily.


 0 kommentar(er)
0 kommentar(er)
